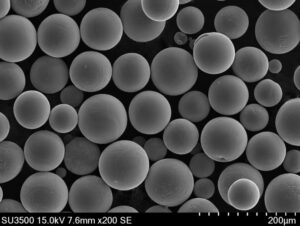
nickel based powder, tiny metallic particles with a multitude of uses, play a crucial role in various industries. But how exactly are these versatile materials produced? Buckle up, because we’re about to embark on a journey exploring the fascinating realm of nickel based powder production methods and delve into their applications, properties, and much more.
The Main Production Methods of nickel based powder
Nickel powders can be created through various methods, each with its own advantages and limitations. Here are some of the most common techniques:
Carbonyl Process: This method involves reacting nickel with carbon monoxide to form nickel carbonyl gas, which then decomposes at controlled temperatures to form spherical, high-purity nickel powder. Imagine nickel atoms hitching a ride on carbon monoxide molecules, only to be gently nudged off at a specific temperature to create a cloud of tiny, uniform nickel spheres. This process is known for its excellent control over particle size and shape, making it ideal for applications demanding high precision.
Water Atomization: In this method, molten nickel is forced through a high-pressure water jet, breaking it up into fine droplets that solidify into irregular-shaped particles. Think of pouring molten nickel through a powerful showerhead, and the resulting spray solidifies into a collection of nickel powder grains. This method is cost-effective and suitable for large-scale production, but the particle size and shape are less controlled compared to the carbonyl process.
Electrolytic Deposition: This method involves using an electric current to extract nickel ions from a solution and deposit them onto a cathode, forming nickel flakes. Picture a nickel-rich solution where, under the influence of electricity, nickel ions are drawn towards a negatively charged surface, gradually building up layer by layer to form thin, plate-like nickel particles. This method offers good control over particle purity but results in non-spherical shapes, potentially impacting flowability and packing density.
Reduction of Nickel Salts: In this method, nickel compounds like nickel oxide or nickel sulfate are reduced using a reducing agent, such as hydrogen, to form nickel powder. Imagine taking nickel locked away in a compound, and using hydrogen as a key to unlock it, transforming it into tiny nickel particles. This method is less common but can be used to produce specific nickel alloys or powders with tailored properties.
Gas Atomization: This method is similar to water atomization, but instead of water, an inert gas like nitrogen is used to break up the molten metal. This results in cleaner and more spherical particles compared to water atomization, but at a higher cost. Think of replacing the water showerhead with a nitrogen one, yielding a cleaner and more uniform spray of nickel droplets that solidify into powder.
These are just a few of the main methods for producing nickel powders. The choice of method depends on factors such as the desired powder properties, cost, and production scale.
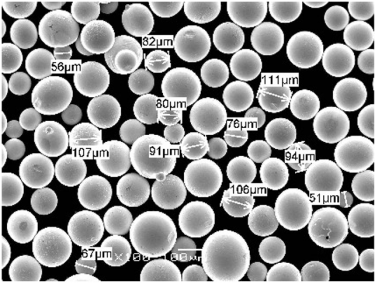
Diverse Landscape of Nickel Powders
Nickel powders come in a variety of shapes, sizes, and compositions, each tailored for specific applications. Here are some notable examples:
INCO 123: This carbonyl-produced nickel powder is known for its high purity, spherical shape, and excellent flowability. It’s widely used in brazing alloys, battery electrodes, and electronic components.
INCO 255: Another carbonyl powder, INCO 255 offers a coarser particle size compared to INCO 123. This makes it suitable for applications requiring good packing density, such as in electrodes and catalysts.
AZL 64: This water-atomized powder features an irregular shape and a wider particle size distribution. It’s often used in applications where cost-effectiveness is a primary concern, such as in powder metallurgy components and thermal spraying.
NiFe: This powder is an alloy of nickel and iron, commonly produced through reduction of mixed metal oxides. It’s used in various magnetic applications, such as soft magnetic cores and electromagnetic interference shielding.
NiCu: This nickel-copper alloy powder offers improved corrosion resistance compared to pure nickel. It’s used in brazing alloys, electronic components, and wear-resistant coatings.
Spherical Nickel Powders: These powders, often produced through carbonyl or gas atomization, boast near-perfect spherical shapes. This makes them ideal for additive manufacturing techniques like 3D printing, where consistent flow and packing are crucial.
Electrolytic Nickel Powders: These powders, characterized by their flaky shape, are used in battery electrodes and catalysts. The high surface area of the flakes enhances their interaction with other materials, leading to improved performance.
Nickel-Based Superalloy Powders: These complex alloys, often containing additional elements like chromium, cobalt, and aluminum, are produced through various methods like gas atomization or plasma atomization. They offer exceptional high-temperature strength and are used in demanding
Diving Deeper: Applications, Properties, and Beyond
The applications of nickel powders are as diverse as their production methods and properties. Here’s a glimpse into the remarkable versatility of these tiny materials:
Applications:
- Battery Electrodes: Nickel powders play a vital role in the production of lithium-ion batteries, a technology powering our portable electronics and electric vehicles. Their high electrical conductivity and specific surface area make them ideal for storing and releasing energy efficiently.
- Additive Manufacturing (3D Printing): Spherical nickel powders are increasingly used in 3D printing to create complex, near-net-shape components for various industries, including aerospace, automotive, and medical. Their excellent flowability and packing density enable precise layer-by-layer deposition, leading to the creation of intricate objects with desired properties.
- Electroplating: Nickel powders are used to create electroplating baths, a technique for coating other materials with a thin layer of nickel. This coating improves corrosion resistance, conductivity, and wear resistance, finding applications in various sectors like automotive, electronics, and jewelry.
- Brazing Alloys: Nickel powders are incorporated into brazing alloys, used to join metal components using a filler metal that melts at a lower temperature than the base metals. These alloys offer excellent strength, ductility, and corrosion resistance, making them crucial for various applications in the aerospace, automotive, and construction industries.
- Catalysts: Nickel powders, due to their high surface area and catalytic properties, are used in various chemical reactions. They can accelerate reaction rates and improve efficiency in processes like hydrogenation, hydrocracking, and reforming, playing a significant role in the chemical and petroleum industries.
- Thermal Spraying: Nickel powders are used in thermal spraying techniques like plasma spraying and high-velocity oxy-fuel (HVOF) spraying to create protective coatings on various surfaces. These coatings enhance wear resistance, corrosion resistance, and thermal properties, extending the lifespan and improving the performance of components in diverse industries like power generation, oil and gas, and aerospace.
Properties:
The properties of nickel powders significantly influence their suitability for various applications. Here are some key characteristics to consider:
- Particle Size and Distribution: The size and distribution of nickel powder particles impact factors like flowability, packing density, and surface area. Finer powders offer higher surface area but may exhibit poorer flowability, while coarser powders flow better but have lower surface area.
- Shape: The shape of nickel powder particles, ranging from spherical to irregular, influences packing density, flowability, and performance in specific applications. Spherical particles offer better packing density and flowability, while irregular shapes may provide improved mechanical interlocking in certain applications.
- Purity: The purity of nickel powder refers to the percentage of nickel present and the level of impurities. High-purity powders are often used in applications demanding high performance and minimal contamination, such as in electronics and battery electrodes.
- Surface Area: The surface area of nickel powder particles plays a crucial role in applications like catalysis and electrochemistry. Higher surface area provides more sites for reactions to occur, enhancing their effectiveness.

Choosing the Right Nickel Powder Production Method
Selecting the most suitable nickel powder production method hinges on understanding the specific needs of your application and carefully weighing the advantages and limitations of each technique. Here’s a comprehensive guide to help you navigate this crucial decision:
Identifying Key Application Requirements:
The first step involves pinpointing the critical requirements of your intended application. Consider the following factors:
- Desired particle size and distribution: Finer powders offer higher surface area but may pose flowability challenges, while coarser powders exhibit better flow but have lower surface area.
- Shape: Spherical shapes generally offer superior packing density and flowability, while irregular shapes might be preferred for applications where mechanical interlocking is crucial.
- Purity: High-purity powders are essential for applications demanding minimal contamination, such as in electronics and battery electrodes.
- Cost: Production methods like carbonyl process offer high purity and control but come at a higher cost, whereas water atomization is more cost-effective but yields less precise particle characteristics.
- Production volume: If large-scale production is necessary, water atomization might be the preferred choice due to its cost-effectiveness and scalability.
Delving into the Merits and Demerits of Each Method:
Now, let’s delve deeper into the pros and cons of each prominent nickel powder production method:
- Carbonyl Process:
Pros: * Exceptionally high purity * Tight control over particle size and shape (spherical) * Excellent flowability and packing density
Cons: * Highest cost among common methods * Complex and energy-intensive process
- Water Atomization:
Pros: * Most cost-effective method * Suitable for high-volume production
Cons: * Less control over particle size and shape (irregular) * May contain impurities due to the water used
- Electrolytic Deposition:
Pros: * Good control over purity * Environmentally friendly process
Cons: * Non-spherical particle shape, impacting flowability * Limited production volume compared to other methods
- Reduction of Nickel Salts:
Pros: * Enables production of specific nickel alloys or powders with tailored properties
Cons: * Less common method with limited availability * May require additional processing steps
- Gas Atomization:
Pros: * Cleaner and more spherical particles compared to water atomization * Offers good control over particle size and shape
Cons: * Higher cost than water atomization but lower than carbonyl process
3. Striking the Perfect Balance:
Carefully weigh the advantages and disadvantages of each method against your specific application requirements. Consider factors like:
- Budgetary constraints: If cost is a primary concern, water atomization might be the most viable option, while high-purity applications in electronics might necessitate the carbonyl process despite its higher cost.
- Production volume: For large-scale production, water atomization is often the preferred choice due to its scalability and cost-effectiveness.
- Desired properties: If achieving a specific particle size, shape, or purity is crucial, the choice might be narrowed down to methods offering the necessary level of control.
Remember, there’s no single “best” method; the optimal choice hinges on your unique application needs and priorities. By understanding the characteristics, advantages, and limitations of each production method, you can make an informed decision that ensures the resulting nickel powder possesses the desired properties for your specific application.
know more 3D printing processes
Frequently Asked Questions (Supplemental)
1) Which production route should I choose for AM‑grade nickel based powder?
- Gas atomization (argon or nitrogen) is the default for LPBF/DED due to high sphericity and clean surfaces; plasma atomization or PREP can further reduce satellites for premium feedstocks. Carbonyl powders are ultra‑pure but often too fine/porous for LPBF unless reprocessed.
2) What PSD is recommended for different AM and PM processes?
- LPBF/SLM: 15–45 μm (tight PSD, high sphericity)
- EBM: 45–106 μm
- DED/blown powder: 45–150 μm
- Binder jetting/MIM: 10–25 μm with tight fines control
3) How do interstitials (O/N/H) affect nickel based powder performance?
- Elevated interstitials reduce ductility and can raise porosity and cracking risk in AM. Typical targets for Ni superalloy powders: O ≤0.04 wt%, N ≤0.01 wt%, H ≤0.001 wt% (verify with inert gas fusion). For catalytic/electrochemical uses, surface chemistry may be tailored differently.
4) Can I reuse nickel based powder in LPBF without compromising quality?
- Yes, with a sieve step (e.g., 63 or 53 μm), magnet and de‑spatter removal, blend‑back with virgin powder, and monitoring PSD, flow, apparent/tap density, and O/N/H. Many facilities qualify 6–10 cycles before full refresh based on mechanical property drift and CT/NDE analytics.
5) What distinguishes carbonyl nickel powder from atomized nickel powders?
- Carbonyl nickel offers extremely high purity and very fine, often spongy/spherical particles used in catalysts, MIM binders, and electrolytic applications. Gas‑/plasma‑atomized powders offer better sphericity and flow for AM and thermal spray; water‑atomized powders are cost‑effective for PM parts but less spherical.
2025 Industry Trends and Data
- Powder passports: Aerospace/energy RFQs increasingly mandate digital passports logging chemistry, PSD, O/N/H, inclusion ratings, reuse counts, and build linkage.
- Sustainability: Argon recirculation, heat recovery on towers, and 20–40% certified recycled content are spreading among nickel based powder suppliers with EPDs.
- Productivity: Multi‑laser LPBF, improved gas‑flow ducts, and AI scan optimization deliver +10–25% build‑rate gains on Ni alloys while maintaining density.
- Safety and compliance: Expanded DHA (dust hazard analysis) for combustible metal powders, with real‑time particulate monitoring and ATEX/NFPA‑aligned systems.
- Binder jetting maturation: Playbooks for Ni‑Cr and Ni‑Cu systems achieve ≥99% density post‑HIP with predictable shrinkage, broadening low‑cost complex parts.
| KPI (nickel based powder & AM), 2025 | 2023 Baseline | 2025 Typical/Target | Why it matters | Sources/Notes |
|---|---|---|---|---|
| LPBF density post‑HIP (Ni alloys) | 99.6–99.8% | 99.8–99.95% | Fatigue and leak‑tightness | OEM/peer‑reviewed data |
| Chamber O2 during LPBF (ppm) | ≤1000 | 100–300 | Oxide/soot control | Machine vendor guidance |
| Qualified powder reuse cycles | 4–6 | 6–10 | Cost, ESG | Plant case studies |
| Satellite count (≥5 μm per 100 particles) | 4–6 | 2–3 | Flow, defect reduction | SEM image analysis |
| Recycled content in feedstock | 5–15% | 20–40% | ESG, cost | EPD/LCA reports |
| Binder‑jet final density w/HIP | 98–99% | 99–99.5% | Mechanical reliability | OEM notes |
Standards and references:
- ISO/ASTM 52907 (metal powder characterization), 52904 (LPBF practice): https://www.iso.org
- ASTM B822/B214 (PSD), B212/B213 (density/flow), F3302 (AM process control): https://www.astm.org
- ASM Handbook: Additive Manufacturing; Nickel, Cobalt, and Their Alloys: https://dl.asminternational.org
- NIST AM Bench datasets: https://www.nist.gov/ambench
- NFPA 484 (combustible metals): https://www.nfpa.org
Latest Research Cases
Case Study 1: AI‑Tuned Gas Atomization Cuts Satellites in Ni‑Cr Alloy Powder (2025)
- Background: A powder producer sought better flowability for LPBF superalloy builds without raising atomization cost.
- Solution: Implemented closed‑loop argon recirculation with O2/H2O scrubbing and high‑speed imaging; ML model controlled gas‑to‑metal ratio and nozzle pressure.
- Results: Satellite count −35%; D50 CV reduced from 7.0% to 4.3%; Hall flow improved by 12%; LPBF customer reported porosity −0.2% absolute and 9% faster spreading.
Case Study 2: Binder‑Jetted Ni‑Cu Heat Exchanger Cores Achieve Near‑Wrought Leak Rates (2024)
- Background: A chemical OEM needed corrosion‑resistant cores with thin fins at lower cost than LPBF.
- Solution: Fine Ni‑Cu powder (D50 ≈ 18–22 μm), tuned debind/sinter with carbon control, followed by HIP; powder passport and SPC on shrinkage.
- Results: Final density 99.2–99.4%; helium leak rate <1×10⁻⁹ mbar·L/s; unit cost −17% at 2k units/year versus machined brazed assembly.
Expert Opinions
- Dr. Todd Palmer, Professor of Materials Science, Penn State CIMP‑3D
- Viewpoint: “In nickel based powder AM, interstitial control and gas‑flow dynamics dominate defect formation—optimize these before fine‑tuning scan vectors.”
- Prof. Ian Gibson, Additive Manufacturing Scholar, University of Texas at Arlington
- Viewpoint: “Process selection should match downstream finishing: LPBF for fine features and internal channels; binder jetting for cost‑effective complexity with HIP.”
- Dr. Martina Zimmermann, Head of Additive Materials, Fraunhofer IWM
- Viewpoint: “Digital powder passports linked to in‑situ monitoring are now standard practice for regulated Ni superalloy parts.”
Affiliation links:
- Penn State CIMP‑3D: https://www.cimp-3d.psu.edu
- University of Texas at Arlington: https://www.uta.edu
- Fraunhofer IWM: https://www.iwm.fraunhofer.de
Practical Tools/Resources
- Standards/QC: ISO/ASTM 52907; ASTM B822/B214 (PSD), B212/B213 (density/flow), F3302; NFPA 484 for combustible metals
- Metrology: LECO O/N/H analyzers (https://www.leco.com); SEM for morphology/satellites; CT and dye‑penetrant for defect/NDE
- Simulation: Ansys Additive, Simufact Additive for scan strategy and distortion; Thermo‑Calc/DICTRA for phase predictions; nTopology for lattice/heat‑exchange design
- Databases: Senvol Database (https://senvol.com/database); MatWeb (https://www.matweb.com); NIST AM Bench datasets
- ESG/traceability: EPD templates and Responsible Minerals Initiative (RMAP): https://www.responsiblemineralsinitiative.org
Last updated: 2025-08-22
Changelog: Added 5 supplemental FAQs; inserted 2025 trend KPI table with standards; provided two case studies (AI‑tuned gas atomization; binder‑jet Ni‑Cu cores); added expert viewpoints with affiliations; compiled standards, metrology, simulation, and ESG resources for nickel based powder.
Next review date & triggers: 2026-02-01 or earlier if ISO/ASTM/NFPA standards update, major OEMs publish new oxygen/reuse specs for Ni powders, or new datasets on atomization satellite reduction and binder‑jet densification are released.